Biochemical study of particulate methane monooxygenase
<Energy-saving methane conversion and selective methanol production>
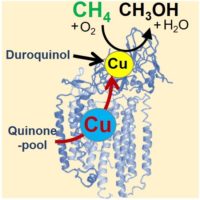
We have shown that methane-oxidizing bacteria can be used as a biocatalyst to convert methane into methanol and biodegradable polymers under normal temperature and pressure conditions (Miyaji et al. 2002). Besides, we have established a method for obtaining high-purity pMMO retaining its enzymatic activity and revealed that pMMO contains only two copper ions (Miyaji et al. 2002). we also revealed the role of copper ions bound to pMMO in the methane oxidation reaction (Miyaji et al. 2019), the formation of a protein complex with methanol dehydrogenase (Myronova et al. 2006), and the electron transfer system from NADH to pMMO in the bacterial membrane (Miyaji et al. 2004). On the other hand, I have been involved in the development of an in-situ gene detection system that can be applied for searching methane-oxidizing bacteria on the deep-sea floor (Fukuba et al. 2007, 2011).
Theoretical and experimental study toward the antioxidant activity of tripeptides
<Rational peptide design for preventing diseases caused by oxidative stress>
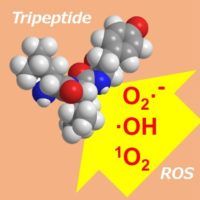
Tripeptides are included in various foods and beverages. Among the tripeptides, some show the activity toward the scavenging of reactive oxygen species (ROS), which depends on their amino acid sequence. ROS causes oxidative stress, which provokes various diseases such as Alzheimer’s disease, Parkinson’s disease, attention-deficit hyperactivity disorder(ADHD), vitiligo, etc. The anti-oxidative tripeptides are expected to be the nutrition keeping our life healthy. For understanding the anti-oxidant activity from the molecular level, electronic structures of tripeptides are analyzed by DFT calculation, and the significant molecular structures generating the anti-oxidant activity are being examined.
Influence of antioxidants on the growth of methane-oxidizing bacteria
<Growth-promoting effect and its application for biocatalyst preparation>
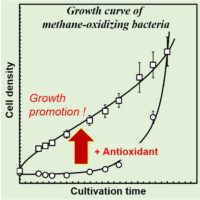
As a result of the enzymatic reaction analysis of pMMO and the analysis of the redox properties of pMMO-bound copper ions by electron spin resonance (ESR), we found that hydrogen peroxide is generated at the copper site of pMMO when the substrate is insufficient (Miyaji et al. 2009). This finding suggests the possibility that ROS generates methane metabolism in the bacterial cells, which may prevent the growth of methane-oxidizing bacteria. This hypothesis motivates us to examine the effect of antioxidants on the growth of methane-oxidizing bacteria. If antioxidants promote the growth of the bacteria, the antioxidants contribute to the short-time preparation of whole-cell biocatalysts for methane conversion.
Regio- and enantioselective alcohol productions by lower-alkane oxidation
<Non-induced fit molecular recognition mechanism of alkane monooxygenases>
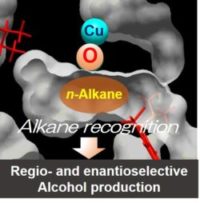
Linear alkane molecules show weak intermolecular forces. Thus, they are difficult to be recognized by a reaction field of catalysts and to be converted to the specific product selectively. However, as a result of the enzyme reaction analysis of pMMO, we revealed that the enzyme shows activity toward the oxidation of linear alkanes (C3-C5) other than methane, and regio- and enantioselectively produces specific alcohol isomers (Miyaji et al. 2011).
Technologies that selectively produces a target substance from resources are required for establishing the no-waste production society, and the sustainable development of the human being. The molecular recognition mechanism of pMMO and its family enzymes would be the fundamental knowledge of catalyst development for selectively producing useful substances from various alkanes that are carbon resources. Therefore, we aimed to clarify how enzymes and catalyst molecules recognize alkane molecules.
By the comparison of the protein structures of family enzymes of pMMO, we have revealed that the protein structure that contributes to the expression of the product selectivity for regio- and stereoisomers of alcohol from linear alkanes (Miyaji et al. 2015). Furthermore, the mechanism of alkane molecule recognition depending on the cavity volume of the enzyme reaction field was clarified (Miyaji et al. 2016). Using the cavity volume of the reaction field as a design index, we have succeeded in giving cytochrome P450 the selectivity from linear octane to 1-octanol (Miyaji et al. 2017). Besides, in the alkane & alkene conversion by zeolites, which have a cavity in its crystal structure as a reaction field, the product selectivity is also expressed depending on the cavity volume, as same as pMMO-family enzymes (Miyaji et al. 2013, 2015, 2016, Iwase et al. 2012, Koyama et al. 2010). Based on these results from the two completely different materials for catalysts, we proposed a new molecular recognition concept of catalysts that the cavity volume is an index for designing a reaction field expressing high product selectivity.
Reactive oxygen generation via the oxidation by tyrosinase
<Model study for the oxidative stress in skin and neuron cells>
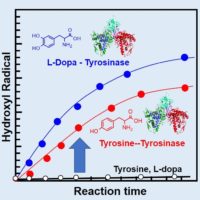
Tyrosinase, which is also the copper-containing enzyme as well as pMMO, is widely present in not only microbes but also animal and plant tissues. For elucidating the mechanism of reactive oxygen species (ROS) generation by tyrosinase, we established the quantitative analysis of various ROS generation during the enzymatic reaction by electron spin resonance-spin trap method. By applying the method, we have clarified that oxidation of tyrosine and L-dopa by tyrosinase produces both hydroxyl radical and singlet oxygen, which are more cytotoxic than hydrogen peroxide. (Miyaji et al. 2016). This result has been applied to the investigation of the vitiligo disease caused by the tyrosinase oxidation of the whitening cosmetic ingredient (Miyaji et al. 2017, Gabe et al. 2018), and the mechanism of water decomposition resulting in the generation of hydroxyl radicals by ultrasound at frequencies used for medical diagnosis (Miyaji et al. 2017).
